VanGenes Preservation Tube
VanGenes® Disposable Virus Sampling Swab and Tube
VanGenes® Disposable Virus Sampling Swab and Tube
Used for virus sample collection, transportation and storage. The main part of the product is a collection tube with preservation solution(not sterile). Two types are provided: non-inactivated and inactivated versions.
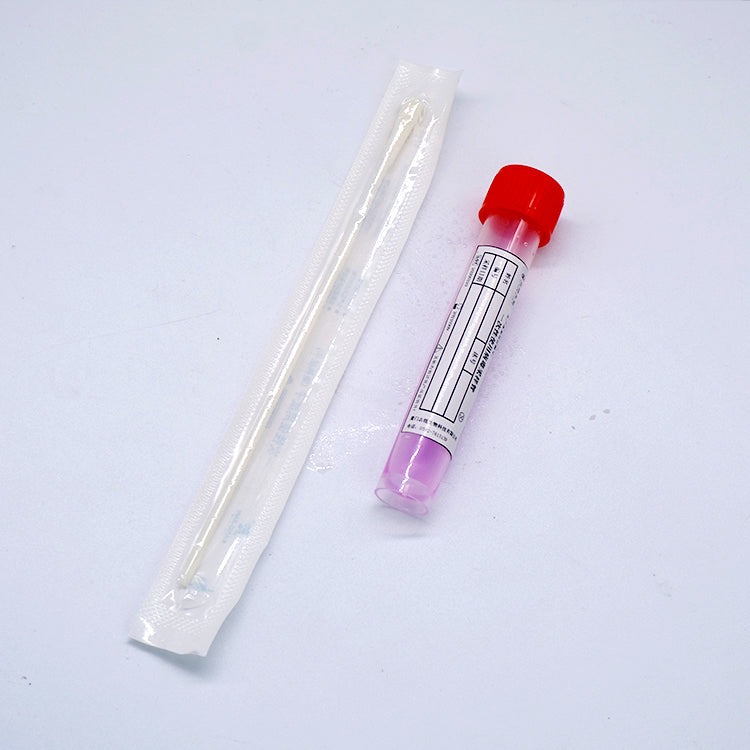
VanGenes Disposable Virus Sampling Tube | VTM Kit | Viral Transport Medias
Overview

VanGenes disposable virus sampling tube is used for virus sample collection, transportation and storage. The main part of the product is a collection tube with preservation solution(not sterile). Two types are available: non-inactivated or inactivated.
Advantage
-
Non-inactivated type:
- Keeps Virus Active: Hank's balanced hydrochloric acid solution is added to the preservation solution with virus-stabilizing components such as FBS.
- Broad Spectrum Antibiotics: The preservation solution has broad-spectrum antibacterial and antifungal effects.
-
Inactivated type:
Safety & Stability: The preservation solution with inactivation function contains GITC, Tris-HCl, EDTA, Triton X-100. This product can process inactivation immediately at the time of sampling while maintaining the stability of nucleic acid in virus samples.
Specifications
| Sample Type | Swab |
|---|---|
| Volume | 3ml(non-inactivated type)/ 6ml(inactivated type)/ 12ml(inactivated type) |
| Material | Plastic |
| Reagents |
For non-inactivited type: Hank's solution, gentamicin, amphotericin, FBS, cryoprotectant, biological buffer For inactivated type: GITC, Tris-HCl, EDTA, Triton X-100 |
| Storage Temperature | Ambient temperature (4~25℃) |
| Working Temperature | Preserve the virus sample 48 hours at 2~8℃, for long term below -70℃ |
| Shelf Life | 12 monthes |
Catalog
| Product&Volume Type | Catalog Number |
|---|---|
| VanGenes disposable virus sampling tube 3ml non-inactivated | ZX-VTM10-3.0(non-inactivated) |
| VanGenes disposable virus sampling tube 6ml inactivated | ZX-VTM10-6.0(inactivated) |
| VanGenes disposable virus sampling tube 12ml inactivated | ZX-VTM10-12.0(inactivated) |
Certificate
| Registration Number | Fujian Xiamen class I medical device record number: 20200164 |
|---|---|
| Registration Type | Registration certificate for class I Medical Device of the People's Republic of China |
| Registered Specification | ZX-VTM10-3.0(non-inactivated), ZX-VTM10-6.0(inactivated), ZX-VTM10-12.0(inactivated) |



